 Quicklime (or calcium oxide or burnt lime) is obtained by calcining pure limestone at temperature above 9000C. This highly reactive product is essential to many industrial processes. Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate.
Quicklime (or calcium oxide or burnt lime) is obtained by calcining pure limestone at temperature above 9000C. This highly reactive product is essential to many industrial processes. Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate.
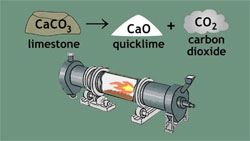 The quicklime is not stable and, when cooled, will spontaneously react with CO2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless slaked with water to set as lime plaster or lime mortar.
The quicklime is not stable and, when cooled, will spontaneously react with CO2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless slaked with water to set as lime plaster or lime mortar.
Quicklime
Quicklime (or calcium oxide or burnt lime) is obtained by calcining pure limestone at temperature above 9000C.ViewMore...
Quick Lime Powder
Lime Powder is free from grits and impurities. This product is being manufactured in our most highy automated machine under close supervision of highly qualified and trained staff.ViewMore...
Hydrated Lime
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. ViewMore..